Miscibility and phase separation of polymer blends has long been studied with the goal of understanding the specific interactions that affect blending. This is especially important for polymers because large macromolecules limit the entropic contribution to the free energy of mixing allowing even weak interactions to play a significant role in miscibility. Understanding how chemical structure affects specific interactions in polymer blends is important in developing blending models that can predict miscibility and blend properties from their individual constituents. We seek a better understanding of blending interactions in polymers and methods to locally tune and control the enthalpic interaction of a blend.
Electric fields are one potential route to tuning the miscibility of polymer blends. Several key energy technologies such as polymer solar cells and batteries involve polymer blends sandwiched between two electrodes such that the application of electric fields are part of the device’s application. Organic photovoltaics have electrode spacings typically of order only 100 nm so that even a modest applied voltage of 1 V easily leads to electric field strengths of 10 MV/m.
How electric fields can modify the miscibility of blends has been an open question dating back to Debye in the 1960s. There are conflicting reports on both the magnitude and direction that the phase separation temperature T_s can shift in the presence of electric fields. Theoretical understanding of the phenomenon has been hampered by the lack of experimental data with unambiguously large shifts in T_s with increasing electric field strength E outside of experimental error.
Characterization of Phase Separation of Polystyrene / Poly(vinyl methyl ether) Blends Using Fluorescence
Annika Kriisa, Sung S. Park, and Connie B. Roth,
Journal of Polymer Science, Part B: Polymer Physics 2012, 50, 250-256.
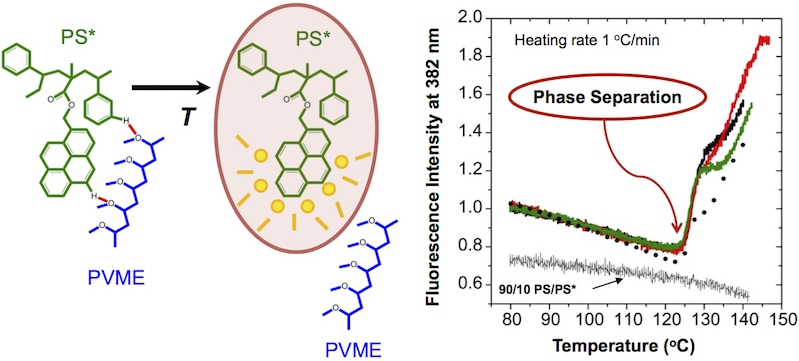
Building on previous fluorescence studies, we developed a method to reliably and reproducibly measure the phase separation temperature T_s of polystyrene (PS) / poly(vinyl methyl ether) (PVME) blends. Using this method, we have characterized for the first time changes in the fluorescence emission spectra of pyrene and anthracene dyes covalently bonded to PS chains upon phase separation from PVME. The specific chemical structure of the fluorescent labels is found to affect the measured phase separation temperature T_s, with fluorophores covalently attached in closer proximity to the PS backbone identifying phase separation a few degrees earlier. The sharp increase in fluorescence intensity upon phase separation that occurs for all fluorophores with little change in spectral shape is consistent with a mechanism of static fluorescence quenching resulting from the specific interaction with a nearby quenching molecular unit. Based on work that has identified a weak hydrogen bond occurring between the aromatic hydrogens of PS and the ether oxygen of PVME, we believe a similar weak hydrogen bond is likely occurring between the PVME oxygen and the aromatic dyes providing a local (few nanometer) sensitivity to phase separation. Our observations indicate that fluorescence is sensitive to the early initiation of phase separation. In comparing similar dyes, fluorophores in which the dye is covalently bonded closer to the PS backbone denote phase separation on average 3 °C earlier than those fluorophores in which the dye is located further away on a covalent tether. As the PVME component moves further away from the PS component upon phase separation, local breaking of these weak hydrogen bonds connecting the PVME oxygen with the aromatic rings of PS and the aromatic dyes, leads to the observed increase in fluorescence intensity.
Electric Fields Enhance Miscibility of Polystyrene / Poly(vinyl methyl ether) Blends
Annika Kriisa and Connie B. Roth,
Journal of Chemical Physics 2014, 141, 134908.
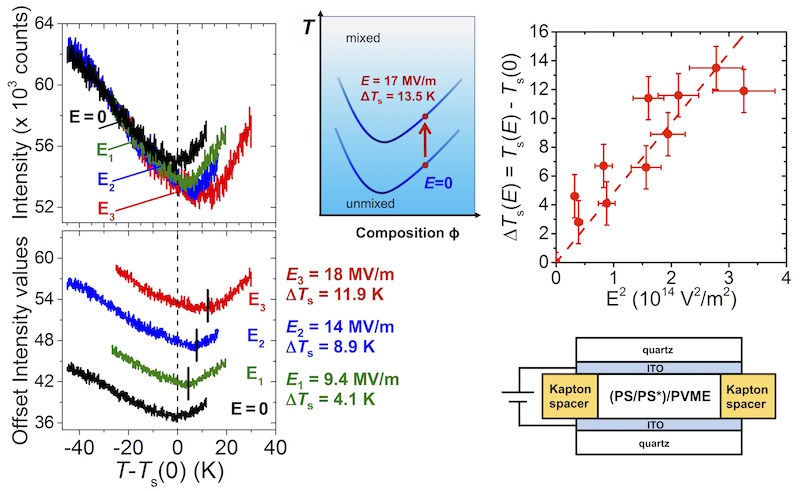
Using the fluorescence method we developed containing trace levels of pyrene-labeled PS, we have reliably demonstrated how the miscibility of PS/PVME blends changes in the presence of uniform electric fields. We have observed large shifts in the phase separation temperature T_s that is reliably and reproducibly well outside of experimental error. For electric field strengths of 17 MV/m, we have measured shifts in T_s of up to 13.5 ± 1.4 K for a 50/50 PS/PVME mixture, some of the largest absolute shifts every reported. We have also demonstrated that the effect is completely reversible with the blend returning to the same T_s value after the electric field has been removed. Our shift in T_s with electric field strength (ΔT_s/E^2) showing enhanced mixing in the presence of electric fields is comparable to other experimental studies in the field on small molecule mixtures, but is still much larger than can be explained theoretically. To understand the phenomenon theoretically, the free energy contribution of electric fields to blend miscibility must be identified. Our experimental results demonstrating increased miscibility with increasing electric field strength E suggests that the term accounting for the dielectric contrast between different components dominates, suppressing concentration fluctuations parallel to the electric field direction and hence the formation of dielectric interfaces between domains during phase separation. Although this reasoning qualitatively explains the direction of the T_s(E) shifts, quantitative agreement with the magnitude of the shift ΔT_s/E^2 is still lacking theoretically.
Jumping In and Out of the Phase Diagram Using Electric Fields: Time Scale for Remixing of Polystyrene/Poly(vinyl methyl ether) Blends
Annika Kriisa and Connie B. Roth,
ACS Macro Letters 2019, 8, 188-192.
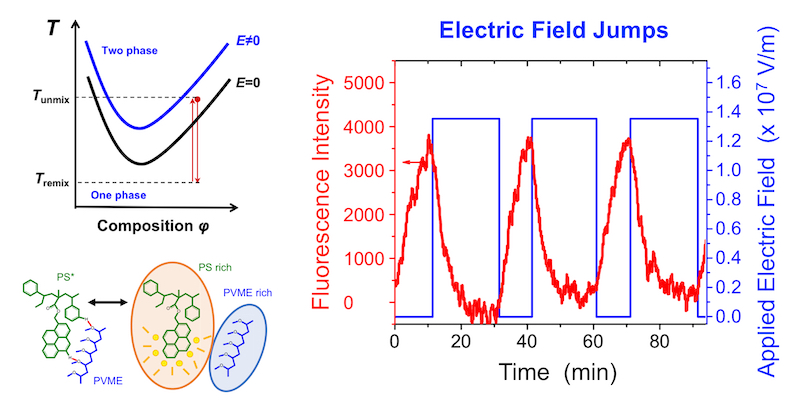
We also demonstrate that electric field can be used as a control knob to repeatedly jump PS/PVME blends from the one-phase to two-phase region by simply turning on and off the electric field when the blend is held at a fixed temperature near the phase boundary. This confirms that electric fields are shifting the miscibility transition. The kinetics of the early stages of phase separation was also investigated, where the remixing time scale τ(T) with and without electric fields were well fit by a Vogel-Fulcher-Tammann expression consistent with a mobility-limited process. Given the prevalence of advanced technologies such as photovoltaics, batteries, and sensors with built-in electrodes requiring idealized blend morphologies, electric field manipulation of phase behavior could have significant design benefits.
